What is Vanillin in Food? Types, Production, Uses, and Differences with Vanilla extract

Vanilla extract | Market | Production | Uses | Safety | FAQs
Vanillin, with the chemical formula C8H8O3, is the world’s most used flavoring agent in food and beverages. It is the vanilla extract alternative that provides a similar smell and taste of vanilla extract. It is both naturally occurring and synthetically produced.
Besides derived from vanilla bean, there are another two types of vanillin available on the market: the artificial one made from chemical synthesis (share more than 90% market) and the natural one comes from biosynthesis.
Difference between Vanillin and Vanilla extract?
Vanillin is a pure ingredient, while vanilla extract is a natural product composed of more than 200 compounds and vanillin is the primary component in it.
Vanilla bean or vanilla pod is the edible fruit and the commercial part of the orchid family. There are more than 150 varieties of vanilla orchids that grow around the world, but only Vanilla planifolia and Vanilla tahitensis are used commercially. (1)
Vanilla extract can be obtained by extraction with a solvent (usually ethanol) from the vanilla pod. Its unique aroma and flavor cannot be replaced by the artificial one.
Vanillin market
The current global annual demand for vanillin is about 20,000 MT. Due to the development of the food industry, its demand may still increase.
Its rough distribution of applications as follows: 60% as a food additive, 30% in the production of flavors & fragrances and cosmetics, 5% used in pharmaceuticals, and the rest as a feed additive.
Low output of natural vanillin
The production areas of vanilla beans in the world are mainly concentrated in Madagascar, Indonesia, and Comoros. The annual output is around 2000 MT/Year.
Due to the limited planting area of vanilla, the labor-intensive work of cultivation, artificial pollination, harvesting, extraction and purification, plus the climate and other factors, making it difficult to increase the production.
Vanillin is present in the form of free molecule or combined with glucoside in vanilla pod. The content is about 1%–2% w/w in cured vanilla pods (2). That’s to say, only around 20-40 MT/Year natural vanillin is available. This number accounts for only about 0.1-2% of its total production in the world.
The price of natural vanillin is extremely expensive, and it can be 300 times higher than the price of synthetic one. That’s why its replacement – the chemical synthesized and biosynthesized vanillin come to the market.
The disadvantages of synthetic vanillin
Due to the high price, natural vanillin is only used in a small application with special demand, and the chemical synthetic type as a substitute takes a large part in the market.
However, the synthetic vanillin cannot compare with the natural one in the aromas although it also has a creamy scent of vanilla extract but the later has a strong creamy flavor. Plus, the manufacturing process is easy to cause environmental pollution.
Meanwhile, the supervision of the use of artificial food additives in many countries has been strengthened, and people’s awareness of food safety has improved, which leads to the increasing market demand for natural vanillin.
Promising biosynthesis vanillin
The microbiological methods are mainly based on the raw materials eugenol (ex Clove oil) and ferulic acid, with the advantages of a higher conversion rate, environmentally friendly and safety.
Such biologically produced vanillin is also considered as natural vanillin according to the regulations of natural flavors from the FDA (3) and EU (4).
How is Vanillin Made?
Generally, vanillin can be made from the following three manufacturing processes: extraction from vanilla bean, chemical synthesis and microbiological fermentation.
Natural extraction method
This is the traditional and oldest way, extracted from vanilla beans (orchid pods), but as mentioned above, the manufacturing process is costly and the yield is low.
Vanillin in mature vanilla beans mainly exists in the form of glucovanillin (vanillin glucoside), which can be hydrolyzed to produce vanillin with β-glucoside hydrolase.
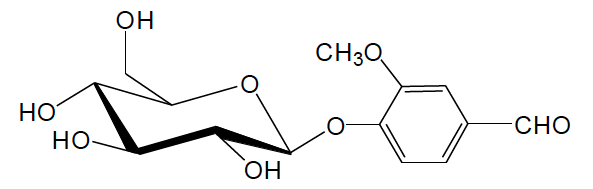
Chemical structure of vanillin glucoside
This manufacturing process has become a popular way to obtain high-quality natural vanillin in recent years, and its unique aroma is difficult to duplicate by the artificial one.
Chemical synthesis
The chemical synthetic methods (about 10 methods available) are the major process of manufacturing vanillin in the global market. It produces vanillin with a high yield, mainly using petrochemical precursor guaiacol, lignin or eugenol as raw materials.
The following are three main used processes.
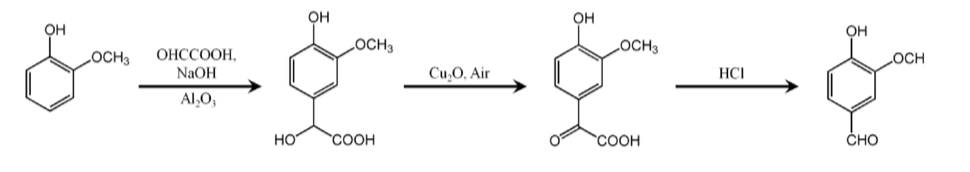
Guaiacol + glyoxylic acid
Under alkaline conditions, guaiacol (made from pyrocatechol) reacts with glyoxylic acid to generate 3-methoxy-4-hydroxyphenylacetic acid, and then undergo oxidative decarboxylation and acidification reactions to synthesize vanillin.
The following is the brief reaction equation:
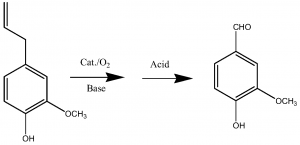
Eugenol
Obtain a vanillin salt by reacting eugenol with oxygen in the presence of a strong base and a catalyst, and then convert the salt to vanillin by reacting it with an acid (5). This method was came up by the China top manufacturer – Jiaxing Zhonghua Chemical Co., Ltd. in 2014.
Here the brief reaction equation:
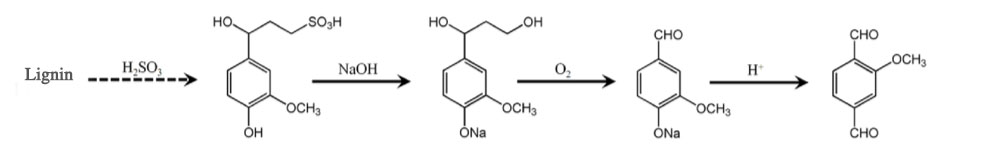
Lignin
Lignin is a renewable resource that can be obtained from various sources. It has a high content in waste wood, straw and pulp waste liquid, mainly in the form of sodium lignosulfonate.
Sodium lignosulfonate is hydrolyzed under alkaline conditions and then goes through oxidative acidification to obtain vanillin.
Here is the brief reaction equation:
By the way, eugenol is also used as raw material to biosynthesis vanillin. (Keep reading and it will be mentioned later)
Biosynthesis
The natural sources of eugenol (ex clove oil) and ferulic acid are commonly used as substrates, and microorganisms such as yeasts, fungi, and bacteria as production hosts to achieve the bioconversion.
The quantity fermented from ferulic acid and eugenol accounts for about 5% of the world’s output. As its quality (aroma) is close to natural vanillin, it is favored by the top food and beverage manufacturers.
The global market demand for vanillin made from these two sources continues to grow and has become more and more popular in the high-end market.
The current annual demand is 500 to 600 tons, and its demand is mainly from the world Well-known fragrance and flavor companies.
Clove oil
Eugenol naturally exists in a variety of essential oils. Its content is high in clove oil, bay leaf oil and clove basil oil. Eugenol from clove oil is mainly used as the raw material in the market.
Ferulic acid
Ferulic acid is one of the most abundant phenolic compounds in nature. It can be found in the cell walls of rice bran, wheat bran, corn bran, and sugar beet crops.
The following brief remanufacturing process is from Solvay:
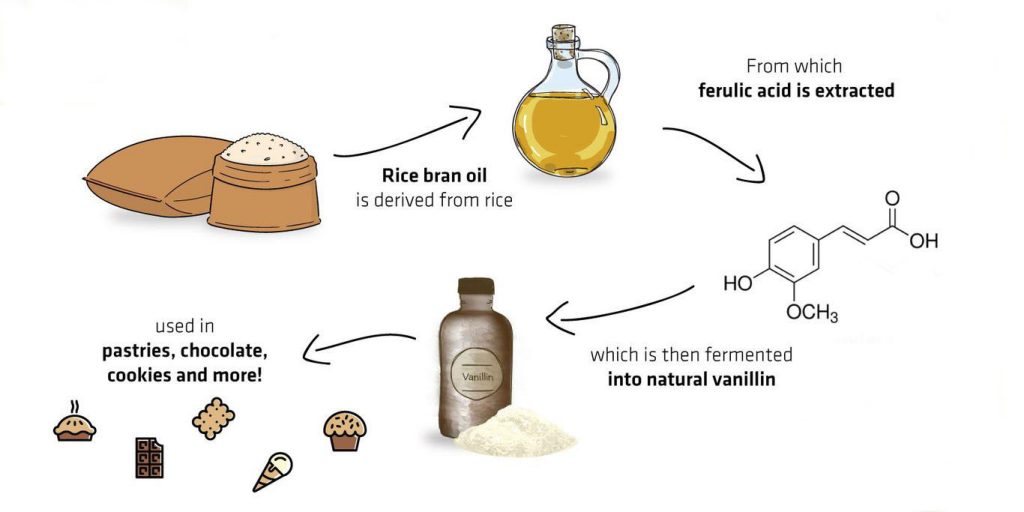
Image Source
It has a chemical structure similar to vanillin, and involves fewer reactions in bioconversation, which is beneficial to increase the conversion rate. Therefore, ferulic acid is an ideal raw material for microbial fermentation.
Properties
An organic compound, a white crystalline powder with a creamy scent of vanilla in smell and taste. Easily affected by light, and gradually oxidized in the air.
| Other names | Vanillaldehyde, Vanillic aldehyde, Imitation vanilla |
| Iupac name | 4-Hydroxy-3-methoxybenzaldehyde |
| CAS number | 121-33-5 |
| Chemical formula | C8H8O3 |
| Molecular weight | 152.15 |
| Melting point | Between 81°C to 83°C |
| FEMA number | 3107 |
| PH value | 4.3, 10g/L water, 20 °C |
Solubility
Slightly soluble in water (1 g/100 mL, 20 ℃), easily soluble in organic solvents such as ethanol, glacial acetic acid and oils.
Structure
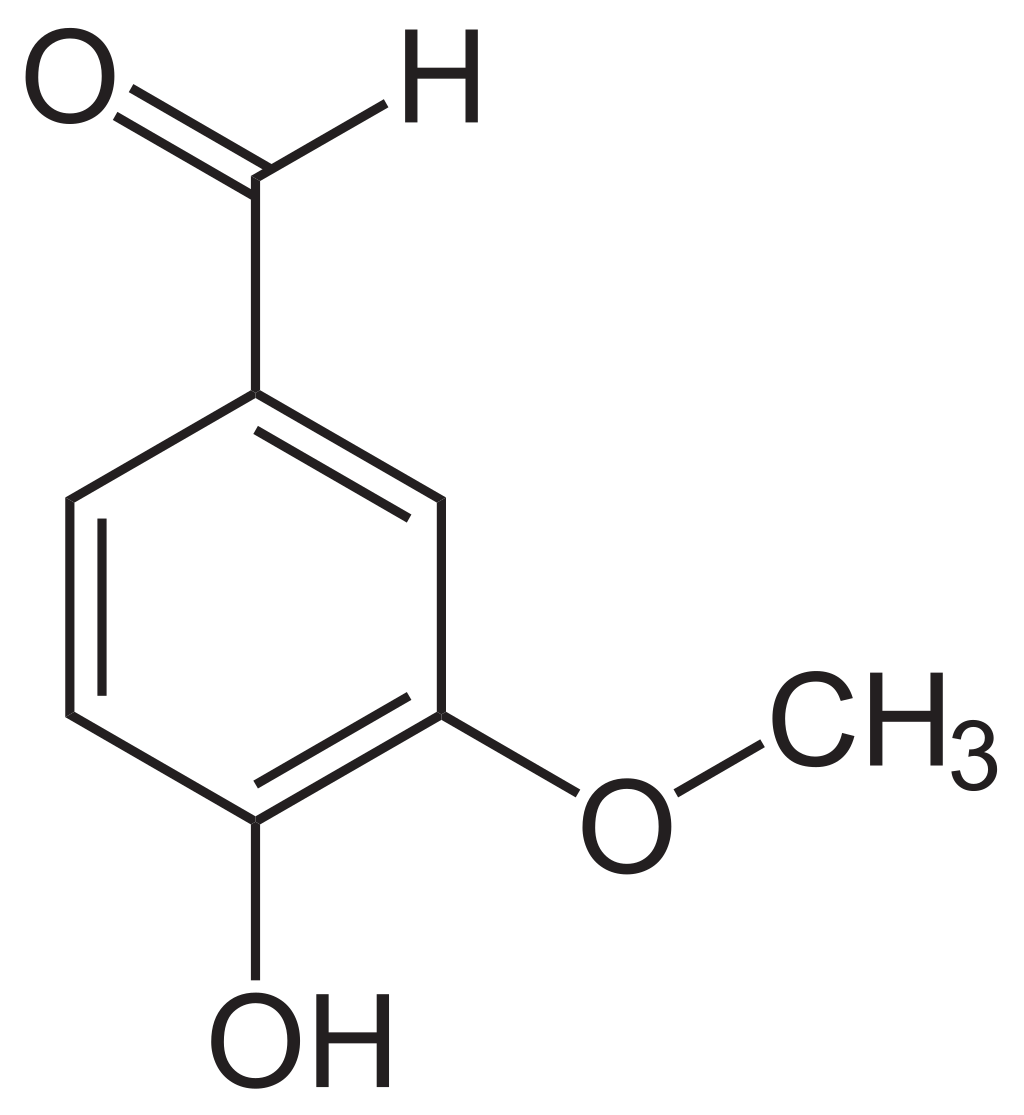
Image Source
It is a phenolic aldehyde, its functional groups include aromatic ring, aldehyde group, hydroxyl phenolic, and methoxy group.
What’re the Uses of Vanillin?
It is the world’s largest used flavors and widely used in various production of foods, toothpastes, perfume, cosmetics & personal care products, and pharmaceuticals.
Food
Flavoring Agent
Vanillin is often used as a flavoring agent to boost milk flavor in sweet food, such as in the following food list:
- Candy
- Ice cream
- Pastry
- Dairy products
- Chocolate
- Bakery (biscuits, baking mixes, cookies, waffles, muffins, cakes)
- Soft drinks, coffee drinks
- Confectionery
- Sauces
- Baby formulas
Vanillin is also used to formulate a variety of flavors and fragrances, for example, in the flavors of pineapple, cherry, banana, cocoa, fruit blends and etc.,
Since the flavor of ethyl vanillin is stronger than vanillin and can last for a long time, vanillin is often used together with ethyl vanillin.
Masking Agent
It can mask the bitterness from proteins (e.g. pea protein, whey protein) or high intensity sweeteners (e.g. stevia). Therefore it can be used in high protein foods designed for weight management and sports nutrition.
Pharmaceutical
In pharmaceutical uses, vanillin is used to mask unpleasant odors or improve tastes. It is mainly used as a masking agent.
Cosmetics
It can be added to cosmetics, perfumes, soap, and toothpaste to make them smell.
Others
It can also be used for rubbers and plastics as well.
Is Vanillin Safe to Eat?
Yes, it almost has no side effects and its safety has been approved by the FDA, EFSA and JECFA as a food flavor in a lot of food.
FDA
Vanillin is an synthetic flavoring substance and adjuvant that is Generally Recognized As Safe (GRAS). (6)
EFSA
Vanillin (FL-no: 05.018) is authorized for use as a flavouring agent in the EU according to Annex I to Regulation (EU) No 1334/2008 (7). Its safety used as a favouring agent was re-evaluated by the EFSA in 2008. EFSA concluded “No safety concern at estimated levels of intake as flavouring substance”. (8)
JECFA
Its ADI (acceptable daily intake) “0-10 mg/kg bw” was set in 1967 and in safety re-evaluation JECFA concluded that “No safety concern at current levels of intake when used as a flavouring agent.” in 2019 (9)
Frequently asked questions
Is it Halal and Kosher?
Yes, three types of vanillin (natural, artificial chemical synthesized and biosynthesized (ex clove oil and ferulic acid) are all halal & kosher complying with the policy of Muslims and Jewish dietary regulations.
Is it Gluten free?
Yes, it is gluten free that complies with the FDA’s definition of gluten free, that it does not contain wheat, rye, barley, or crossbreeds of these grains.
Is it Vegan?
Yes, it is vegan as the manufacturing process without the use of animal matter or products derived from animal origin. So it is suitable for vegetarians.
Conclusion
Now you may have a knowledge of the flavor – Vanillin, from the following aspects:
- Difference with vanilla extract
- Three production processes: from vanilla pod, chemical synthesis and microbiological biosynthesis.
- Uses as a flavoring agent and masking agent in food
- Safety
- FAQs
What kinds of food labels have you found this ingredient in? Or if you have any questions or remarks about this additive, feel free to let me know in the comments.



Is it possible to provide the author of this article for the purpose of citation? Thank you.
you’ll find the author end of the page