What Is Benzoic Acid (E210) & Why Used Less than Sodium Benzoate In Food?

Source | Production | Properties | Uses | Safety | Side effects
You may already know benzoic acid as a chemical. Yes, this ingredient is commonly used as an industrial intermediate. But have you heard of its uses in food? How is it made? How many of its derivatives? Why it is less common in food than sodium benzoate? What’re the common uses besides in food? …You’ll find all these answers in this article (keep reading).
Benzoic acid (the European food additive number E210), is an aromatic carboxylic acid not commonly used for food preservation due to the slight solubility in water. It is usually produced to its soluble salts – widely used preservatives, sodium benzoate (E211) and potassium benzoate (E212, not common, to reduce the sodium intake).
Natural source
Benzoic acid can be naturally found in free or combined forms in apples, cranberries, prunes, plums, and so on.
How is Benzoic Acid made?
There are two types of benzoic acid, one is the natural form extracted from plants and can be used as a flavor with the FEMA No. 2131. Another one is the most used one, obtained from toluene synthesis and here we would like to introduce the brief manufacturing process of this one.
Toluene oxidation
Benzoic acid is largely commercially produced by liquid-phase oxidation of toluene (from petrochemical processing) with oxygen in the presence of a cobalt catalyst.
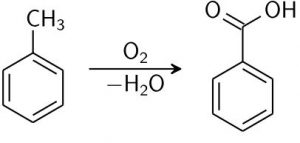
Image Source
If for food and pharmaceutical applications, other manufacturing processes (e.g. sublimation, recrystallization and neutralization) need to be further performed to purify crude benzoic acid as it may contain by-products such as benzaldehyde, benzyl alcohol and benzyl benzoate. (1)
Other methods
Food grade benzoic acid can also be manufactured from the following two methods mentioned by the FDA (2):
- molten phthalic anhydride with a zinc oxide catalyst
- hydrolysis of benzotrichloride
By the way, benzoic acid can also be produced from sodium benzoate with HCL.
Specification
| Other Names |
|
| CAS Number | 65-85-0 |
| Chemical formula | C7H6O2 or C6H5COOH |
| Molar Mass | 122.123 g·mol−1 |
| Boiling Point | 250 °C (482 °F; 523 K) |
| Melting Point | 122 °C (252 °F; 395 K) |
Properties
- A white crystalline flake, needle, powder or granular with a benzoin or benzaldehyde odor.
- Slightly soluble in water while easily soluble in ether, ethanol, dichloromethane, diethyl ether and other organic solvents.
- A weak acid and has an acid taste that will influence the taste and PH of food.
Structure
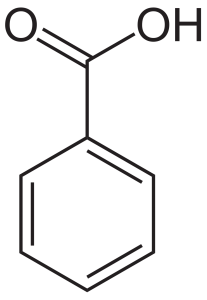
Image Source
The structure is benzene with a carboxylic acid. It is made of the functional group carboxyl and aromatic benzene ring. There can be many derivatives generated by the reactions of these two functional groups with other chemicals.
1. Carboxylic acid
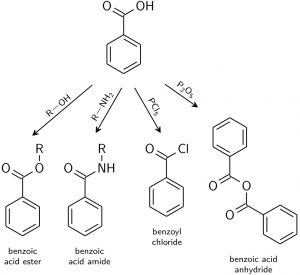
Image Source
Carboxylic acid is easy to react with other groups, such as the following reactions:
- Producing sodium benzoate: with sodium bicarbonate or sodium hydroxide.
- Esterification: with alcohols to form esters, such as methyl, ethyl, butyl or hexyl benzoate used in artificial flavors, fragrances and personal care products. Or through the reaction of two molecules benzoic acid to form benzoic acid anhydride.
- Amidation reaction: with amino forms benzoic acid amides.
- Replacement reaction: OH in carboxylic can be replaced by chloride to obtain benzoyl chloride.
- Reduction: benzyl alcohol.
- Others: such as to make benzaldehyde.
2. Aromatic benzene ring
Aromatic benzene ring can also react with other substances :
- Nitration forms m-nitrobenzoic acid.
- Hydroxylation to produce salicylic acid. By the way, benzoic acid and salicylic acid can be used together to treat skin irritation and inflammation.(3)
PKa
PKa of benzoic acid is 4.2. The pKa value is used to exhibit the acidity or the strength of an acid, as weak acids partially dissociate in water and reach a condition of equilibrium.
How to calculate the PH of Benzoic Acid solution?
Benzoic acid is a weak acid and it dissociates proton and benzoate ions after its reaction with water.
dissociate equilibrium
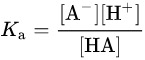
Ka value
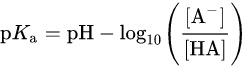
relationship between pka and ph
Above is the dissociation equation of benzoic acid and the relationship between PKa and PH, HA stands for benzoic acid, A- stands for benzoate ion.
Step 1: Suppose the concentration of total HA is 0.001 mol/L.
There can be more benzoic acid soluble than 0.001 mol/L if at the temperature 25°C. To be exactly, the mol concentration would be n=solubility/molecular formula, that’s say n=3.44/122.123 = 0.028mol/L. Here using 0.001 mol/L just to make it easy.
Step 2: Write the ionization equilibrium equation as follows:
HA(aq)+H2O(l)⇌A−(aq)+H3O+(aq)
- [H3O+] is the Hydronium concentration
- [A−] is the conjugate base concentration
- [HA] is the benzoic acid concentration
The concentration of [A−] and [H3O+] is the same. And we suppose the concentration is X. And the final concentration of HA in solution is 0.001 – X.
Step 3: Then the equation can be written X*X/0.001-X = Ka.
As 0.001-X can be similar to 0.001, so we can get the X value 10-3.6, so the PH value is 3.6.
Slightly polarity and Solubility
Benzoic acid is structured by a smaller polar carboxylic acid group attached to a big, non-polar benzene ring. As it has a large hydrophobic benzene ring, it is considered slightly polar.
Generally, polar compounds can dissolve in polar solvents. So that’s why benzoic acid is slightly soluble in water (polar) and dissolved in the non-polar solvent like ether and MTBE (Methyl tert-butyl ether).
Although ethanol is polar, benzoic acid can dissolve in it as both will have an esterification reaction. This is an exception.
The solubility of benzoic acid in water or in other solvents generally related to the polar of solvents, the solution temperature, and other solutions, like NaOH or HCl.
OK, let’s see the details as follows.
1. Water solubility
Benzoic acid is a weak acid, so it only partially dissociate in water and thus it is slightly soluble in water, with the solubility 3.44 g/L in the temperature 25 °C. It can dissolve in hot water as the high temperature promotes the dissociation to its conjugate base benzoate ion, and with the solubility 56.31 g/L in 100 °C.
2. Solubility in NaOH
Benzoic acid is soluble in NaOH. The reaction mechanism is as follows: NaOH is a strong base, it reacts with the dissociated proton from benzoic acid and then promotes the dissociation of benzoic acid.
This is how NaOH affects its solubility in water.
And if there is enough NaOH, benzoic acid will be totally dissociated and forms sodium benzoate but loses its preserving activity in alkaline conditions.
3. Solubility in HCl
In an acidic medium, such as in HCl, benzoic acid becomes more insoluble. As the increase of H+ ions will make the equilibrium of dissociation to the left, making it more insoluble than in water.
HA(aq)+H2O(l)⇌A−(aq)+H3O+(aq)
What are the Uses of Benzoic Acid?
Apart from its application in foods and beverages, benzoic acid can also be added to cosmetics and pharmaceuticals for preservation. However, its main use is as a precursor for the synthesis of organic chemicals in various industries, such as in perfumes, plastics, dyes and so on.
As JECFA said, 30 years ago, the percentages and the uses of benzoic acid in the market were as follows (4):
- 60% was used to produce phenol
- 30% for caprolactam in nylon fibers
- 5% for the production of sodium, potassium and calcium benzoates
- 3% for benzoyl chloride
- 2% for alkyd resins, benzoate esters and others
Currently, the uses do not change much, benzoic acid is also used as a feed additive and as an intermediate in pharmaceuticals and plastics manufacturing.
Let’s have a closer look at its different uses.
Food
Food grade benzoic acid functions as a preservative that decreases the growth of microorganisms in acidic food. It is mostly active on yeasts, and also on molds and bacteria. Its antimicrobial activity would be good at the pH ranges from 2.5 to 4.0.
Why Benzoic Acid is less common in food?
But its uses in food and beverage are not common compared with sodium benzoate and potassium benzoate, as three main issues (especially the solubility):
- Solubility: slightly soluble in water, not easy to use
- PH: It is an acid that will influence the PH of food and drink
- Taste: Its solution has an acid taste, will affect the taste
That’s why it is always made to its salt – sodium benzoate although it is benzoic acid that has the antimicrobial activity.
Typical use levels of benzoic acid and sodium benzoate in food are between 0.05–0.1%.
The following food may contain it:
- Fruit juice
- Pickles
- Jams
- Sparkling drinks
- Soda
Cosmetics
Benzoic acid can be used as a bulking, masking and preservative (5) in a variety of cosmetics and personal care products, including:
- Skincare products: facial moisturizer, sunscreen
- Mouthwash
Industrial Intermediates
The industrial use is closely related to the aromatic ring or the carboxyl group on it. Its common uses are the carboxylic acid reaction with other substances, such as for phenol production, plastics and benzyl benzoate.
Phenol
The production of phenol and caprolactam is the most common use for benzoic acid.
Here is the reaction:
C6H5CO2H + 1/2 O2 → C6H5OH + CO2
Phenol can be converted to cyclohexanol, which can be used in nylon synthesis. Phenols can also be used in household cleaners and in mouthwash as a disinfectant.
Plastics
Benzoic acid can be made to benzoate plasticizers, such as glycol-, diethyleneglycol-, and triethyleneglycol esters.
Is Benzoic Acid Safe to Eat?
Yes, its food grade has been approved as a safe ingredient by the U.S. Food and Drug Administration (FDA), European Food Safety Authority (EFSA), as well as Joint FAO/WHO Expert Committee on Food Additives (JECFA).
FDA
Benzoic acid is generally recognized as safe (GRAS) that can be used as an antimicrobial agent, flavoring agent and adjuvant in food with the maximum usage of 0.1%. It can also be safely used in feed as a preservative or acidifying agent. (6) (7)
EFSA
Benzoic acid (E210) is listed in Commission Regulation (EU) No 231/2012 as an authorised food additive and categorized in “Additives other than colours and sweeteners” (8).
Safety re-evaluation in 2016
Its safety was re-evaluated together with its salts and EFSA derived an acceptable daily intake (ADI) of 5 mg/kg bw per day in 2016.
Approved uses
Same with that of sodium benzoate. (9)
UK Food Standards Agency
Categorized in “Others” (10)
Food Standards Australia New Zealand
It is an approved ingredient in Australia and New Zealand with the code number 210. (11)
JECFA
Function Class: food additives, antimicrobial preservative. (12)
Acceptable daily intake: ADI “5 mg/kg bw” set in 1996 and no safety concern as a flavouring agent evaluated in 2002. (13)
What’re possible Side Effects of Benzoic Acid?
When it comes to the side effects of benzoic acid, it usually refers to its allergies and whether it converts to benzene which is a carcinogen. Let’s see the details:
- Allergies: it may cause irritation symptoms such as rash, redness, or even burning feeling from eye and skin contact, and also by inhalation. (14)
- Benzene: FDA doesn’t mention that benzoic acid forms to benzene while sodium and potassium benzoate does. (15)
Is it safe during pregnancy?
Benzoic acid is generally considered safe for pregnant, but better to consult with your doctor for your condition of use.
Conclusion
Now you may have a good knowledge of the preservative – benzoic acid (E210), from the following aspects:
- Natural source
- Production process
- Properties
- Uses
- Safety
- Possible side effects
If you have any questions or remarks about this additive, feel free to let me know in the comments.


