What is Calcium Stearate (E470a) in Food and its common Uses?

Composition | Production | Uses | Safety | FAQs
Calcium stearate, the calcium salt of stearic acid derived from animal fats & oils or vegetable oils, with the chemical formula Ca(C18H35O2)2.
It is a synthetic ingredient that can be used as an anti-caking agent, release agent, lubricant, binder, emulsifier, and thickener in food with the European food additive number E470a. It also can be used in cosmetics, pharmaceuticals, plastics and other applications.
What is it made of?
From the USP definition, calcium stearate consists of calcium stearate and calcium palmitate. Also, it contains 9.0%-10.5% calcium oxide (CaO). (1)
How is Calcium Stearate Made?
It can be produced as a white precipitate by the reaction between calcium chloride and sodium stearate in aqueous solution. The following is the chemical equation:
CaCl2 + 2C17H35COONa → (C17H35COO)2Ca + 2NaCl
It can also be made by reacting stearic acid with calcium oxide (2). The following is the chemical equation:
2C17H35COOH + CaO → (C17H35COO)2Ca + H2O
Source
The main raw material – stearic acid is a naturally occurring saturated fatty acid existing in the form of glycerides in animal fats & oils and vegetable oils.
- Common animal source: mutton tallow, beef tallow, and lard.
- Common vegetable source: cocoa butter, soybean oil, palm oil, coconut oil, olive oil and corn oil.
Most stearic acid used in the production of calcium stearate is from vegetable oils, so it can be called vegetable calcium stearate in this way.
Specification
| Other names | Calcium salts of fatty acids, Calcium distearate, Calcium soap, Calcium octadecanoate |
| CAS number | 1592-23-0 |
| Chemical formula | Ca(C18H35O2)2 |
| Molecular weight | 607.03 |
| Melting point | 155 °C (311 °F; 428 K) |
Properties
Appearance
White to yellowish-white waxy powder or dispersions, with a slight, characteristic fatty odor, and a grease touch.
Solubility
Like magnesium stearate, it is sparingly soluble in water (solubility 0.004 g/100 mL at 25 °C), while the other two metallic stearates, sodium and potassium stearate are soluble in water.
Slightly soluble in hot alcohol, hot vegetable and mineral oils. (3)
Why is it insoluble in water?
Divalent Ca2+ can bind two non-polar fatty acid molecules, while Na+ and K+ can only bind to one fatty acid as a monovalent ion. Two fatty acid molecules prevent Ca2+ interact with water molecules, thus making it insoluble in water.
Structure
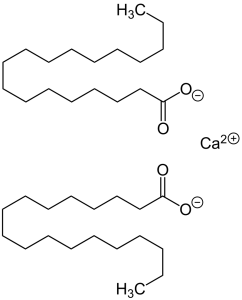
Image Source
It is made up of two stearate anions and a calcium cation. Totally there are 36 carbons in length of molecule structure.
What are the Uses of Calcium stearate?
Calcium stearate is mainly used as an anti-caking agent in food, cosmetics and pharmaceuticals for its lubricating and water-repelling properties.
Food
It can be used as a solid-phase lubricant to prevent ingredients and finished products from sticking caused by absorbing moisture.
In bread, it is a dough conditioner that functions as a free-flowing agent, and is commonly used together with other dough softeners such as mono- and diglycerides. (4)
The following food list may contain it:
- Bakery
- Calcium supplements
- Mints
- Soft & hard candies
- Fats and oils
- Meat products
- Fish products
- Snack foods
Cosmetics
It is generally used for its lubricating properties. It maintains emulsions from separating into oil and water phases in cosmetics and personal care products.
Pharmaceuticals
It is an excipient that can be used as a mold-release agent (to help machines run fast) in the manufacturing of pharmaceutical tablets and capsules.
Plastics
Calcium stearate is used as a lubricant, release agent and an acid scavenger (to neutralize hydrochloric acid) in the manufacturing of plastics, e.g. vinyl polychloride (PVC) and polyethylene (PE).
Other uses
It can also be used in coating, paper, rubber, concrete, textile, an antifoaming agent and so on.
Is Calcium Stearate Safe to Eat?
Calcium stearate almost has no side effects when used as a food additive. It is generally recognized as safe (GRAS) in food with no limitation other than current good manufacturing practice by the FDA. It can be used as a flavoring agent & adjuvant, a lubricant & release agent, and a stabilizer and thickener. (5)
In Europe, calcium salts of fatty acids (E470a) are approved ingredients and its safety as food additives have been re-evaluated by the EFSA in 2018.
The EFSA concluded that no safety concern at the reported uses and use levels, and no need to establish a numerical acceptable daily intake (ADI) of it. (6)
Frequently asked questions
Is it Natural?
No, Calcium stearate is not a natural ingredient as it is made from chemical synthesis.
Is it Halal and Kosher?
Yes, it is halal and kosher if the raw material – stearic acid derived from vegetable oils.
Is it Gluten free?
Yes, calcium stearate complies with the FDA’s definition of gluten free, that it does not contain wheat, rye, barley, or crossbreeds of these grains.
Is it Vegan?
Yes, most used today in food products is vegetable calcium stearate. And it is considered vegan as the manufacturing process without the use of animal matter or products derived from animal origin.
But stearic acid also can be produced from animal fats and oils, therefore, vegetarians should avoid this source.
Is it Dairy free?
Yes, although it has calcium, it is not made from milk and do not contain dairy.
Conclusion
Now you may have an understanding of the Salts of Fatty Acids – Calcium Stearate (E470a), from its production; uses in food, cosmetics, pharmaceutical and plastics; the approved safety and so on.
What do you think of this anticaking agent? Let me know in the comments.


How much quantity is used in food powders of vegetables as Tamarind ,Ginger Garlic etc