What is L-Tartaric Acid (E334) in Wine? Four Types, Uses, Safety, Side Effects

Four Types | Uses | In wine | Safety | Side effects | FAQs
Tartaric acid, a naturally occurring organic acid commonly found in grapes and used in winemaking. It has a sour taste and can be used as an acidulant, flavoring agent, antioxidant and chelating agent in food with the European food additive number E334. Generally, it is safe, natural or synthetic, vegan, halal, kosher and gluten-free.
Four types of Tartaric Acid
Tartaric acid is a dihydroxyl derivative of succinic acid. As there are two asymmetric carbon atoms in its molecule, so it exists in four different stereoisomers: L, D, DL and Meso. The L and DL forms are commonly used as food additives.
1. L-Tartaric Acid
When it comes to tartaric acid, we’re mostly talking about L-(+)-tartaric acid, the dextro form which with the CAS number 87-69-4 and E number E334. It is used widely like other acidulants (citric acid and malic acid) for its high solubility, strong tart taste (about 1.2 to 1.3 times that of citric acid), and its stable salts.
Natural Source
L tartaric acid is widely present in nature and mostly in the form of potassium, calcium and magnesium salts or free state. It can be found in a variety of plant fruits such tamarinds, grapes, bananas. Tartaric acid is another major grape acid, along with malic acid. But unlike malic acid, its concentration does not decline much during the grape ripening process.
How is it Made?
Generally, there are three production methods to obtain this ingredient: by-products of winemaking/grape, enzyme process, and microbial fermentation. The first method is commonly used among European manufacturers for the abundant raw materials – grapes, while China is the biggest manufacturers for the second method.
1. Winemaking byproduct
During the winemaking process, a part of tartaric acid is precipitated in the form of cream of tartar crystals (potassium bitartrate), or more lovingly, “wine diamonds”. The first step of production is the recrystallization of cream of tartar as its solubility increases along with the rising temperature.
Then reacting it with calcium hydroxide and calcium chloride to produce calcium tartrate (insoluble).
And finally, L tartaric acid is obtained by reacting calcium tartrate with sulfuric acid. It can also be extracted from the grape.
For your better understanding, the following are the four steps reaction equations:
- Cream of tartar (Recrystallization) → KHC4H4O6
- 2KHC4H4O6 + Ca(OH)2 → CaC4H4O6 + K2C4H4O6 + 2H2O
- K2C4H4O6 + CaCl2 → CaC4H4O6 + 2KCl
- CaC4H4O6 + H2SO4 → CaSO4 + C4H6O6
It is shown in the European Parliament that the total output quantity in 2016 was around 35, 000MT, and 86% was produced in Europe. (1)
2. Enzyme process
The enzyme method is the mainstream commercial production method of L tartaric acid in China due to the high purity, high conversion efficiency, and safety. The manufacturing flow chart as follows:
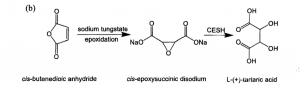
- Maleic anhydride is oxidized to sodium cis-epoxysuccinate by hydrogen peroxide.
- Sodium cis-epoxysuccinate is hydrolyzed to tartaric acid using cis-epoxysuccinate hydrolase (ESH)
3. Fermentation method
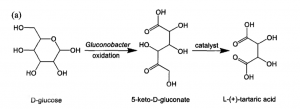
Using glucose as raw material, through microbial fermentation, the glucose is oxidized to 5-keto-D-gluconate (5-KGA), and then catalyzed to L- tartaric acid.
2. D-Tartaric Acid
It is not the natural form and seldom used in food, also known as D(-)-tartaric acid with the CAS number 147-71-7, it can be obtained by chiral split DL tartaric acid.
3. DL-Tartaric Acid
Racemic tartaric acid is a mixture of equal amounts of L and D, the CAS number 133-37-9. Its manufacturing flow chart is similar to that of producing L tartaric acid by enzyme but without the enzyme used in the hydrolysis process. DL tartaric acid can be used as a PH regulator, chelating agent, and also used to produce tartrates.
4. Meso Tartaric Acid
CAS number 205-696-1. It is optically inactive due to internal molecular symmetry.
Specification
|
Appearance |
Colorless or translucent crystalline granular or powder with a sour taste. |
|
Other names |
|
|
CAS number |
13463-67-7 |
|
Chemical formula |
C4H6O6 |
|
Molecular weight |
150.09 |
|
Melting point |
168 °C and 170 °C |
Properties
Solubility
- In water: it is polar and highly water-soluble, 20°C, 139g/100ml (L form) and 18.4g/100ml (DL form)
- In organic solvents: 33g/100ml (L form), sparingly soluble (DL form) in ethanol.
PKa
It is a weak dicarboxylic acid, but stronger than malic and citric acid. L form has two PKa, PKa1 2.98 and PKa2 4.40 at 25°C, respectively.
PH
PH value 3.18 with the concentration 1 mmol/L at 25°C and 2.55 with the concentration of 10 mmol/L. (2)
What are the Uses of Tartaric acid?
Tartaric acid, citric acid and malic acid are the most common organic acids used in food. In general, food grade tartaric acid is used as an acidulant to provide a tart taste for wines, beverages, juices, marmalades, ice cream, sour confectionery, gelatin desserts, jams and jellies.
Why is Tartaric acid used in wine?
Tartaric acid is commonly added in wine making as the natural acidity of grapes is not enough, such as in red, white, and fruit wines. It is the primary acid that influences the taste, balances color, tannins and sweetness, and maintains chemical stability which are important to the mouthfeel in fermentation, barrel aging process and finished wines. Also, it acts as a preservative which prevents the growth of undesirable spoilage bacteria by lowering PH.
Tartaric acid’s solubility in wine varies according to the temperature. It dissociates into two forms, bitartrate (HT–, mainly present at wine pH) and tartrate (T-2). So it presents three forms in wine together with tartaric acid (H2T). Different percentages of these three forms in different pHs.
Usually, at room temperature, it binds with potassium in wine to form the crystalline tartrate – cream of tartar. Maybe you have the experience of finding these small crystals on the cork, or bottom in the wine.
The Raw Material for producing Emulsifiers
It reacts with fatty acid and glycerol to produce the following three food emulsifiers:
- Tartaric acid esters of mono- and diglycerides of fatty acids (TATEM, E472d)
- Mono- and diacetyl tartaric acid esters of mono- and diglycerides of fatty acids (DATEM, E472e)
- Mixed acetic and tartaric acid esters of mono- and diglycerides of fatty acids (MATEM, E472f)
Cosmetics
It functions as a buffering, and masking agent in cosmetic and personal care products. (3)
Pharmaceutical
It can be used as an excipient and used to improve the taste of the effervescent tablets.
Is Tartaric acid Safe to Eat?
Yes, it has been approved as a safe ingredient by U.S. Food and Drug Administration (FDA), European Food Safety Authority (EFSA), as well as Joint FAO/WHO Expert Committee on Food Additives (JECFA).
FDA
L-(+)-tartaric acid obtained from wine making is generally recognized as safe (GRAS) as a direct human food ingredient and can be used as a firming agent, flavor enhancer, flavoring agent, pH control agent and humectant in food with no limitation other than current good manufacturing practice. (4)
EFSA
L-tartaric acid (E334) is listed in Commission Regulation (EU) No 231/2012 as an authorised food additive and categorized in “Additives other than colours and sweeteners” (5).
Approved uses
E334 is listed in the group I which means most of its applications are not specific. The following food may contain with L-tartaric acid:
- Canned or bottled fruit and vegetables
- Jam, jellies and marmalades and sweetened chestnut purée
- Cocoa and Chocolate products
- Fresh (pre-cooked) pasta
- Potato Gnocchi
- Table-top sweeteners in tablets
- Biscuits and rusks and baby foods for infants and young children
UK Food Standards Agency
Categorized in “Others” (6)
Food Standards Australia New Zealand
It is an approved ingredient in Australia and New Zealand with the code number 334. (7)
JECFA
Function Class: food additives, flavouring agent, acid, antioxidant synergist, sequestrant. (8) (9)
Acceptable daily intake: Group ADI “30 mg/kg bw” set in 1977 for it and its salts of sodium, potassium, and potassium–sodium. (10)
What are the Possible Side Effects of Tartaric acid?
It is generally considered safe and there were few side effects reported.
Frequently Asked Questions
Is Tartaric acid Natural?
L form is a plant acid coming from nature and it is natural if manufactured from the byproduct of winemaking or extracted from grapes. It is chemical synthetic if produced from maleic anhydride, and the racemic form is also synthetic.
Is Tartaric acid Halal?
Yes, it is halal. Both L and DL forms comply with the Islamic Law and fulfill the conditions of Halal. And we can find some manufacturers certificated with MUI halal.
Is Tartaric acid Vegan?
Yes, Both its L and DL forms are vegan as animal products are not involved in the manufacturing process. So it is suitable to add in the diet of vegetarians.
Tartaric Acid (TA) vs Citric Acid (CT)?
Numbers of carboxylic acid: TA is a diprotic acid while CT is a triprotic acid.
Food Source: TA is mostly present in grapes whereas citric acid naturally occurs in citrus fruits.
Taste: TA is sourer than CT.
Production: TA is derived from the byproduct of winemaking or synthesis from maleic anhydride while CT is obtained from carbohydrate (from corn) fermentation.
Application: tartaric acid can be the substitute for citric acid in some food uses.
Tartaric Acid vs Cream Of Tartar?
They’re not the same and with different food applications. Cream of tartar, the bitartrate salt of tartaric acid, can be produced by reacting tartaric acid with potassium hydroxide, as it has been partly neutralized so it is less acidic than tartaric acid.
Cream of tartar can be blended with sodium bicarbonate and cornstarch as baking powder in food, such as in cookies and meringues. Due to the very low water solubility (4g/1L,10º C) of cream of tartar, the reaction between the cream of tartar and baking soda will not happen until the temperatures are reached. This maximizes the volume development in the bakery product.
Conclusion
Now I think you may have a good knowledge of the acidulant – Tartaric acid (E334), from the following aspects:
- Four stereoisomers: L, D, DL and Meso forms of tartaric acid.
- Four production processes
- Uses: mainly in wine
- Approved safety
- Possible side effects
- FAQs: is it vegan, halal, and comparison with citric acid and cream of tartar
What kinds of food labels have you found this ingredient in? Let me know in the comments.


