What is Saccharin (E954) in Food & Toothpaste? Uses, Safety, Side effects and More

Types | Production | Uses | Safety | FAQs
Saccharin or saccharine, the oldest but controversial artificial sweetener, which has been used as a low-calorie sweetener and sugar substitute in food and beverage for more than 100 years with the European food additive number E954.
With the advantage of lower price and heat stability (250°C, thus suitable for cooking and baking), it was widely used in food and beverage. However, its food uses are not as popular as it used to be, but its market in toothpaste is big.
China exported around 15,000MT saccharin and its salts in 2019, and the USA imported about 900MT from the global market.
There are three problems with this sweetener:
- Bitter taste
- Considering its association with bladder cancer in studies in the 1970s (although authorities have demonstrated no such relationship in 2000), its application is limited and with the ADI of 15 milligrams per kilogram body weight per day (mg/kg bw/d) (1)
- Environmental pollution from the manufacturing process
Let’s see more about this ingredient. Commonly we say saccharin refers to sodium saccharin, which is the common form of saccharin that is used in food and toothpaste.
Types
Saccharin can be divided into two types: water insoluble and soluble saccharin. The commercial saccharin on the market are mainly its soluble salts, commonly as sodium saccharin, also with a little calcium saccharin and potassium saccharin.
Saccharin
Used less in food, also known as insoluble saccharin or the acid form of saccharin, chemical name o-benzoyl sulfonimide, CAS number 81-07-2, with molecular formula C7H5O3NS, molecular weight 183.18 and melting point 226 to 230 °C.
Sodium saccharin
Available in anhydrous and dihydrated forms, exist in granular, powder, and liquid appearance. Its granular is often used in situations where it needs to be dissolved, and powder is usually used in dry mixes and pharmaceuticals.
| Other names | Sodium saccharine, Benzoic sulfimide, sodium ortho-sulphobenzimide, Saccharin sodium |
| CAS number | 128-44-9 |
| Chemical formula | C7H4NNaO3S·2H2O (dihydrate with 15% moisture) C7H4NNaO3S·2H2O (anhydrous with 6% moisture) |
| Molecular weight | 241.19 (dihydrate), 223.19 (anhydrous) |
Solubility
Freely soluble in water, sparingly soluble in ethanol. It dissociates saccharin anions and sodium ions in water.
Taste
Its sweetness is about 300 times higher than that of sucrose (table sugar). However, it has an unpleasant slightly bitter and metallic aftertaste in the mouth when its concentration exceeds 0.03%. Glycine can be used to mask the bitter aftertaste of saccharin in beverages and beverage bases. (2)
Synergy
Synergistic with other sweeteners to reduce the slight bitter metallic aftertaste. For example, it is commonly blended with aspartame and cyclamate.
Structure
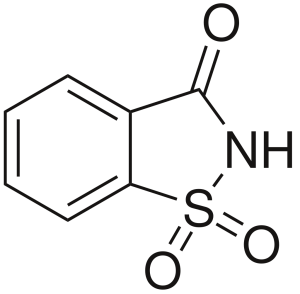
Image Source
The functional group is carboxylic sulfonimide.
Calcium saccharin
Chemical formula C14H8CaN2O6S2·3½H2O, Molecular weight 467.48. It is sometimes used to replace sodium saccharin to reduce sodium intake.
Potassium saccharin
Chemical formula C7H4KNO3S·H2O, Molecular weight 239.77, seldom used in food.
How is Saccharin Made?
Saccharin can be made from a variety of synthetic routes. There are two main manufacturing processes. One is the Remsen-Fahlberg method, the oldest process since its discovery, in which toluene is synthesized by chlorosulfonic acid at first. Another process using phthalic anhydride or methyl anthranilate as a starting material.
Remsen‐Fahlberg process
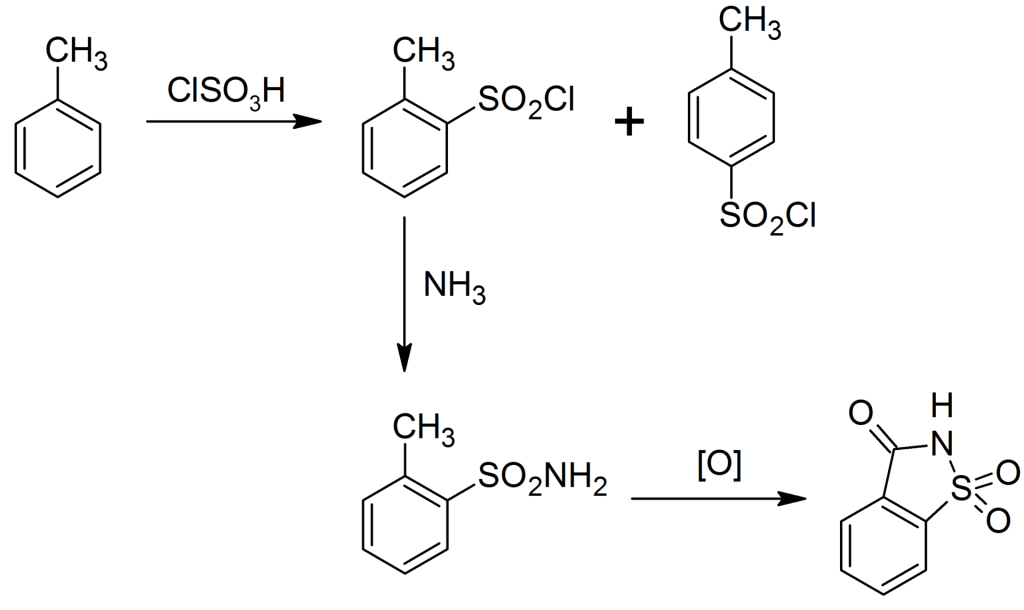
Image Source
In this process, toluene reacts with chlorosulfonic acid to synthesize ortho and para-toluenesulfonyl chloride. Then reacts with ammonia to produce the corresponding toluene sulfonamide.
The ortho-toluene sulfonamide is separated in the solvent of sulfonyl chloride, then oxidized to anthranilic acid, and then heated and cyclized to become saccharin.
Finally react with NaHCO3 to obtain sodium saccharin.
Phthalic anhydride or methyl anthranilate process
The brief process based on methyl anthranilate as follows:
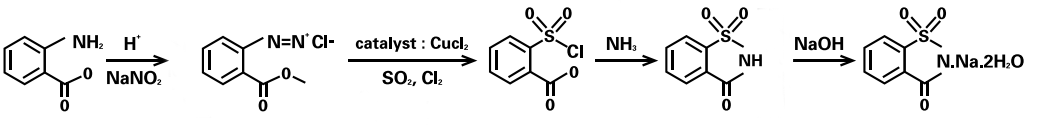
Image Source
Diazotization: react methyl anthranilate with sodium sulfite to form 2-carbomethoxy benzene-diazonium chloride. Sulfonation & oxidation: then react with sulfur dioxide and chlorine to yield 2-carbomethoxy benzene sulfonyl chloride.
Obtaining insoluble saccharin through amidation of the sulfonyl chloride by reacting with ammonia and following acidification.
Finally, the reaction with sodium hydroxide to produce the sodium salt.
What’re the Uses of Saccharin?
Saccharin has been used to reduce calorie and replace sugar in our food and drink for more than 100 years due to the advantages of low cost, synergy with other sweeteners, and stable property. It is also used in toothpaste but not allowed in baby food.
Food
It is commonly used for the following low-calorie and sugar-free products:
- Soft drinks
- Table‐top sweetener packets (you may find it in restaurants or airlines)
- Baked goods
- Chewing gum (saccharin can slowly dissolve in the mouth)
- Dry & canned fruit
- Pickles
- Candy
- Coffee
- Yogurt, desserts, ice-cream, dessert toppings and salad dressings
- Jam, preserves, marmalade
- Vitamin tablets
Table top sweeteners
It is available in tablets in the market and the tablets are made of sodium bicarbonate, sodium saccharin, silicon dioxide, povidone, and modified cellulose gum. (3)
Carbonated soft drinks
Saccharin was widely used to sweeten beverages due to its rapid dissolving in water in earlier years. However, the availability of other low-calorie sweeteners (such as aspartame, acesulfame potassium, sucralose and neotame), allows manufacturers/consumers to have more choices, and thus make saccharin less popular as it once was in soft drinks.
Coca-Cola
The purpose of saccharin in Coca-Cola’s drinks is as a calorie-free sugar substitute. (4)
It was the first used (approved) non-caloric sweetener in the first generation of Diet Coke and made it successful in 1982. (5)
We can find this ingredient in Fanta-Zero (6) and Lilt (7).
Pepsico
Pepsico beverage are almost not sweetened with saccharin except Diet Mtn Dew (8) and you’ll find it is not in its list of non-Caloric Sweeteners, which are composed of aspartame, sucralose, acesulfame k and stevia extract. (9)
Toothpaste
Saccharin is commonly used in personal care products such as toothpastes and mouthwash as a sweetening agent, which will not cause tooth decay.
You can find this additive in the products of Crest, with the functions of better taste, good stability and not contributing to the development of cavities. (10)
Also, it is in some of Colgate’s toothpaste, it is used as a flavor/sweetener to make fluoride and abrasives in toothpaste taste delicious. (11)
Other applications
It can also be used in pharmaceuticals, animal feeds, pesticides, and electroplating.
What’re the Benefits of Saccharin?
Generally, saccharin has the following health benefits:
- Zero Glycemic Index: suitable for diabetics
- No calories
- Reduce dental cavities
- Weight control
Good for diabetics
It is excreted unchanged mostly through urine (12), and does not raise blood sugar (zero glycemic index). So it is safe and appropriate for diabetics.
No calorie
It is a non-carbohydrate sweetener and does not contain any calories.
Tooth friendly
It can be used as a sugar alternative to reduce dental cavities in sugar-free gum & candy for children.
Weight management
Low-calorie sweeteners (e.g. aspartame, saccharin and sucralose) provide sweetness with almost no calories, which is good for weight management. (13)
Is Saccharin Safe to Eat?
Yes, its safety has been approved by the U.S. Food and Drug Administration (FDA), European Food Safety Authority (EFSA), Joint FAO/WHO Expert Committee on Food Additives (JECFA), Health Canada, UK Food Standards Agency (FSA), as well as Food Standards Australia New Zealand (FSANZ).
However, the possible carcinogenicity has limited its use in food from 1970-2000.
FDA
Saccharin and its salts may be safely used as sweetening agents in the following food list (14):
- Beverages, fruit juice drinks, and bases or mixes: not more than 12 mg per fluid ounce.
- As a sugar substitute for cooking or table use: not exceed 20 mg per each expressed teaspoonful of sugar sweetening equivalency.
- Processed foods, not more than 30 mg per serving.
It can also be used to reduce bulk and enhance flavors in chewable vitamin & mineral tablets; retain flavor and physical properties of chewing gum; and enhance the flavor of flavor chips in nonstandardized bakery products.
History
Saccharin was discovered in 1879, but a warning label on saccharin-containing products was necessary after the finding of the link between saccharin consumption and the development of bladder cancer in laboratory rats in the early 1970s. (15)
In 1999, the International Agency for Research on Cancer (IARC) reclassified saccharin and its salts as “not classifiable as to their carcinogenicity to humans (Group 3)” from the conclusion that the mechanism of observed bladder cancers in rats was not relevant to humans. (16)
In May 2000, the U.S. National Toxicology Program (NTP) removed saccharin from its list of “reasonably anticipated to be a human carcinogen” since 1981. (17)
Finally, President Clinton issued the SWEETEST Act to remove the warning label on all products using saccharin on December 21, 2000. (18)
EFSA
Saccharin (E954i), sodium saccharin (E954ii), calcium saccharin (E954iii) and potassium saccharin (E954iv) are listed in Commission Regulation (EU) No 231/2012 as an authorised food additive and categorized in “ Sweeteners” (19).
Approved uses
The following are some of its uses in energy-reduced or/and no added sugar food (20):
- Canned or bottled fruit and vegetables
- Jams, jellies and marmalades
- Cocoa and Chocolate products
- Sauces
- Breakfast cereals
- Table-top sweeteners in powder/liquid/tablets form
- Processed fish and fishery products
Health Canada
The food uses of saccharin had been restricted (only as a table-top sweetener) for a long time in Canada since the 1970s for the reason of its carcinogenicity in laboratory rats. (21)
In the presence of many other artificial sweeteners, saccharin would be another sweetener option for the selection and choice of consumers.
On April 24, 2014, saccharin and its salts were re-authorized and can be added as sweeteners in the following food list (22):
- Breath fresheners
- Unstandardized canned fruit
- Chewing gum
- Unstandardized frozen desserts
- Toppings/topping mixes
- Unstandardized alcoholic liqueurs
- Unstandardized carbonated non-alcoholic beverages unstandardized fruit spreads
The maximum usage of saccharin and its salts are not more than 0.25% except in table-top sweeteners. (23)
JECFA
Function Class: food additives, sweetener. (24)
Acceptable daily intake: ADI “0-5 mg/kg bw” set in 1993. (25)
FSA
Categorized in “Sweeteners” (26)
FSANZ
It is an approved ingredient in Australia and New Zealand with the code number 954. (27)
Frequently asked questions
Is it keto?
Yes, it is keto friendly as it is not a carbohydrate, no effect on blood sugar.
Saccharin vs aspartame vs sucralose?
Taste: saccharin has a bitter taste, while the other two artificial sweeteners don’t.
Heat stability: aspartame is heat unstable and will decompose around 80 °C.
Safety: sucralose has little side effects while another two has several possible health problems.
Price: saccharin is the cheapest among three.
Market share: saccharin is the first generation high intensity sweetener, aspartame is the second and afterward sucralose. Sucralose has been used widely, the market of saccharin and aspartame seems stable.
Is it Halal and Kosher?
Yes, it is kosher and halal, and complies with Muslims and Jewish religious dietary law.
Is it gluten free?
Yes, it is gluten free that complies with the FDA’s definition of gluten free, that it does not contain wheat, rye, barley, or crossbreeds of these grains.
Is it Vegan?
Yes, it is vegan as the raw materials and the manufacturing process without the use of animal matter or products made from animal origin.
Conclusion
Now you may have a knowledge of the artificial sweetener – Saccharin (E954), from the following aspects:
- 4 forms: insoluble saccharin, and its sodium, calcium and potassium salts
- 2 Production processes
- Uses in soft drink, table top sweeteners, toothpaste and etc.
- Benefits
- Safety history in the USA and Canada market.
- Side effects especially of causing bladder cancer in the early years.
- FAQs: is it keto, comparison and differences with other artificial sweeteners.
What kinds of food labels have you found this ingredient in? Or if you have any questions or remarks about this additive, feel free to let me know in the comments.


