What is Sodium Dehydroacetate in food: Uses and Safety

Uses | Production | Properties | Safety
Sodium dehydroacetate (SDHA), the sodium salt of dehydroacetic acid, is a new generation of food preservative after benzoates, parabens and sorbates. SDHA can effectively inhibit the growth of microorganisms such as bacteria, yeast and mold.
This ingredient is commonly used in bread and pastries to improve food stability and thereby extend the shelf life.
What’re the Uses of Sodium Dehydroacetate?
Sodium dehydroacetate is widely used as a preservative in food, cosmetics, and pharmaceutical products.
When dissolved in water, it dissociates dehydroacetic acid, which is an effective preservative but seldom used directly due to its insolubility in water, which limits its use in food and cosmetics applications.
How to use it in food?
Sodium dehydroacetate can be directly added to the food for mixing or premixed with other ingredients first, it can also be made into a solution for food soaking, spraying or surface treatment, depending on the type of food.
The following are the two main food uses: bread and pastries.
Bread
The antimicrobial properties of SDHA inhibit the activity of baker’s yeast, which will lead to the prolonged fermentation time of bread (which uses yeast for increasing volume). At the same time, part of SDHA will be lost in high-temperature baking above 130 ℃.
The microencapsulated SDHA may be used to solve this problem by weakening SDHA’s effects on the fermentation of baker’s yeast, and reduce the loss of this ingredient due to the influence of high temperature.
Pastries
The combination of potassium sorbate and sodium dehydroacetate is commonly used for the preservation of pastries, but the PH of most pastries are alkaline, which leads to the less preservation effect of potassium sorbate.
Glucono delta-lactone can be added in the combination as an acidity regulator, resulting in a better preservation of the two ingredients in pastries.
Cosmetics
It can be used as a preservative to prevent the growth and reproduction of microorganisms in the formulation of cosmetics and personal care products.
The following products you may find it in:
- Lotion
- Bath
- Skin care
- Makeup
Feed
It can also be used for the preservation of feed for livestock and poultry, e.g. feed for pigs and broilers.
How is Sodium Dehydroacetate Made?
The brief manufacturing process of its food grade can be described as follows (1):
- Obtain dehydroacetic acid by polymerizing diketene (this material is also used in the production of Acesulfame potassium)
- neutralization reaction with sodium hydroxide
The following is the general flow chart:
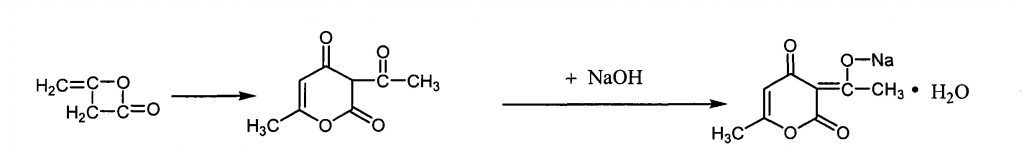
Properties
An odorless, white crystalline powder, with the chemical formula C8H7NaO4·H2O, and molecular weight 208.15.
Structure
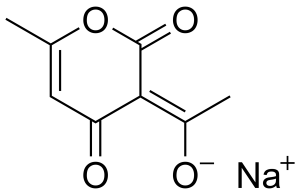
Image Source
Solubility
- Easily soluble in water (1g/3mL), propylene glycol (1g/2 mL) and glycerin (1/7mL).
- Slightly soluble in ethanol (1%) and acetone (0.2%).
Concentration
Sodium dehydroacetate has a broad-spectrum antimicrobial ability on yeasts, molds, and bacteria. And its effective concentration ranges from 0.05% to 0.10%.
Compared with the traditional preservative – sodium benzoate, the inhibitory effect of sodium dehydroacetate:
- on molds is around 40-50 times stronger
- on bacteria 15-20 times stronger inhibitory effect.
PH
Preservatives need a suitable PH value to exhibit its antibacterial effect. The optimal pH range for the common preservatives as follows:
- Benzoic acid & benzoates: 2.5 – 4.0
- Sorbic acid & sorbates: 3.0 to 6.5
- Propionic acid & propionates: 2.5 to 5.0
You can see these preservatives only effective in an acidic atmosphere. However, the antibacterial effect of sodium dehydroacetate is not much affected by the acidity and alkalinity, as it is effective both in acidic and alkaline conditions, but better under acidic conditions.
Safety
When used as a preservative, the safety of dehydroacetic acid and sodium dehydroacetate have been approved by the U.S. Food and Drug Administration (FDA) for cut or peeled squash. (2)
Conclusion
Now you may have a knowledge of the food preservative – Sodium Dehydroacetate, from production, uses, approved safety and comparison with benzoates, sorbates and propionates.
What kinds of food have you found this ingredient in? Let me know in the comments.



can i use SDHA powder in my dry fried meats(pork/chicken) and vegetables?
hi,
The uses should comply with your food law.
It is not common in dry fried meats(pork/chicken) and vegetables.
You can see other preservatives for more information: https://foodadditives.net/preservatives
Hi,
I’ve seen this ingredient used in 2-3 popular brands of tapioca pearls for Boba tea. For example:
https://www.fooducate.com/product/WuFu-Yuan-Black-Tapioca-Pearl/54B868AD-3780-47E0-4F1F-9B53D9029AF4
The article only indicates a very specific FDA approval of the ingredient for cut or peeled squash, but if I understand correctly (https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=25d184619bdc0c352d2d32a9dc93ba0b&ty=HTML&h=L&mc=true&n=pt21.3.175&r=PART) then it is generally approved as a preservative. Right?
I was wondering whether there are any hazards.
Is this ingredient vegan or vegetarian?