What is Sodium Alginate (E401) in food? Properties, Uses, Safety

Production | Properties | Uses | Safety | FAQs
Sodium alginate, the sodium salt of alginic acid extracted from brown seaweed (Phaeophyta) with the European food additive number E401. It is the main form of alginates used in food that can function as a thickener, stabilizer and gelling agent. This ingredient is commonly used to produce heat-stable gels and to impart viscosity in food.
How is Sodium Alginate made?
Alginic acid and its salts (calcium, magnesium and sodium) are naturally present in the cell walls of brown seaweed. The most used species for the production of alginic acid and its salts are Macrocystis, Ascophyllum, Durvillaea, Ecklonia, Laminaria, Lessonia, and Sargassum. (1)
Generally, sodium alginate is made by the reaction of sodium carbonate with alginic acid. The brief manufacturing process are as follows (2):
- Washing seaweed with water and acid to move water-soluble substances, such as sodium chloride, potassium chloride, colors, mannitol and soluble proteins.
- Convert insoluble ingredients (alginic acid and calcium alginate) to soluble sodium alginate by adding sodium carbonate, and then remove cellulose (insoluble) by filtration.
- Convert sodium alginate to alginic acid by adding acid or calcium chloride to form calcium alginate first and then wash with acid.
- Neutralization with sodium carbonate, and followed up by the process of drying, granulation or sieving.
Specification
| Other names |
|
| CAS number | 9005-38-3 |
| Chemical formula | (C6H7NaO6)n |
| Molecular weight | 10 000 -600 000 (typical average) |
Properties
Appearance
White to yellowish brown filamentous, powder or granular, nearly odorless and tasteless.
Structure
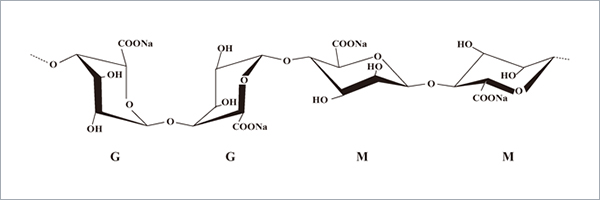
Image Source
Sodium alginate is a polymer of polysaccharide mainly made of α-L-guluronic acid (G unit) and β-D-mannuronic acid (M unit) connected by α ( 1→4) glycosidic bonds.
The ratio of M/G (were related to the species of seaweed and the harvested season) will affect the viscosity, gelling capability and gel strength.
Solubility
Dissolve in hot or cold water to produce a viscous colloidal solution while calcium and magnesium alginate does not soluble insoluble in ethanol.
Viscosity
Generally, the viscosity depends on:
- Degree of polymerization
- The molecular weight of the sodium alginate polymer
- Temperature
- Concentration
- Presence of polyvalent metal cations
This property can be used as a thickener and the viscosity (1% solution) of its food grade can vary from 4 to 1000 mPa·s.
PH
Ph ranges from 4.0-10.0 in its food applications.
Gelling
A heat-stable gel would be formed with acids or multivalent cations, e.g. calcium, copper, and lead ions.
A calcium salt is commonly used to react with a sodium alginate solution to form a gel in which polymers are cross-linked by calcium ions, such as in the production of jelly.
The following image is the gel before and after heating, and we can see the alginate gel is heat stable than the gels of gelatin, carrageenan and agar.
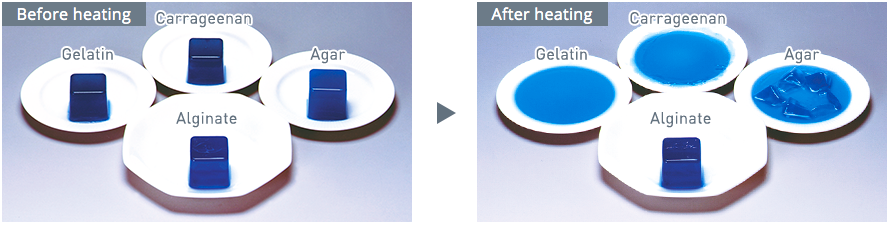
Image Source
Gel synergy: a firmer gel occurs by the combination of sodium alginate with pectin. Such gels are thermo-reversible compared with thermally stable sodium alginate (with calcium ions) gels.
What’re the Uses of Sodium Alginate?
Food grade sodium alginate is widely used in food, especially in dairy-based frozen products (e.g.ice creams, yogurts, puddings, cheese sauce and desserts) because of its properties of thickening, gelling, and stabilizing.
It can be used for the following applications:
Ice cream
It provides a smooth and soft mouthfeel, and also stabilizes the bubbles in frozen ice cream and prevents the formation of sugar crystals.
Spherification
Sodium alginate and calcium chloride or calcium lactate can be used together to shape a liquid into squishy beads or worms as calcium ions can make sodium alginate to gel. This way can encapsulate food color and flavor into the spheres.
Meat
Sodium alginate (with calcium chloride) can be used as a texturizer in meat to retain the water, enhance the elasticity and adhesion of meat products, and improve the freshness of finished products.
The principle is that sodium alginate and calcium ions form a gel of calcium alginate that holds the water in the meat.
Supplements
It can be used in dietary supplements to adjust appetite and energy intake. Alginate–konjac–xanthan polysaccharide complex (PGX) is made by blending sodium alginate, konjac glucomannan and xanthan gum in a specific ratio which is used in fortified foods and supplements.
Feed
It can also be used as a binder in pets products, other non-food-producing animals and fish.
Pharmaceuticals
It can be used as an active ingredient to reduce reflux of gastric content or an excipient in pharmaceutical products. (3)
Is Sodium Alginate Safe to Eat?
Yes, it has been approved safe by the U.S. Food and Drug Administration (FDA) and European Food Safety Authority (EFSA), as well as the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
FDA
Sodium alginate is generally recognized as safe (GRAS) that can be used as a thickener, stabilizer, texturizer and firming agent in (4):
- Condiments and relishes
- Confections and frostings
- Gelatins and puddings
- Hard candy
- Processed fruits and fruit juices
- Other food categories
EFSA
Sodium alginate (E401) is listed in Commission Regulation (EU) No 231/2012 as an authorised food additive and categorized in “ additives other than colours and sweeteners” (5).
Safety re-evaluation in 2017
EFSA conducted the safety revaluation for alginic acid and its salts of sodium, potassium, ammonium and calcium as food additives, and derived the following conclusions (6):
- No safety concern and no need to establish an ADI as an additive used in food.
- The usage for infants and young children should be below therapeutic dosages.
- Inadequate assessment of the safety when used in “dietary foods for special medical purposes and special formulae for infants”and “dietary foods for babies and young children for special medical purposes”.
Approved uses
It is classified into “Group I” with the maximum use levels “quantum satis”, also listed separately and with alginic acid (E400), potassium alginate (E402), ammonium alginate (E403) and calcium alginate (E404). The following food may contain it (7):
- Unflavoured pasteurised cream
- Meat preparations
- Desserts and puddings
- Jam, jellies and marmalades
UK Food Standards Agency
Categorized in “Emulsifiers, stabilizers, thickeners and gelling agents” (8)
Food Standards Australia New Zealand
It is an approved ingredient in Australia and New Zealand with the code number 401. (9)
JECFA
Function Class: food additives, emulsifier, gelling agent, stabilizer, thickener. (10)
Acceptable daily intake: ADI “not specific” set in 1992. (11)
Frequently asked questions
Is it natural?
Yes, sodium alginate is a natural polysaccharide composed of two types of uronic acids.
Is it vegan?
Yes, it is vegan as the raw material used and manufacturing process without the use of animal matter or products derived from animal origin. So it can be added in the food for vegetarians.
Is sodium alginate a hydrocolloid?
Yes, as it can thicken or gel water.
What’re the benefits?
Sodium alginate is a non-digestible carbohydrate and the FDA intends to propose it in the list of dietary fibers. It has the benefits of lowering blood sugar, blood pressure, cholesterol levels and other health effects. (12)
Conclusion
Now you may have a knowledge of the thickener – sodium alginate (E401), from the following aspects:
- Production process: extracted from seaweeds.
- Properties: viscosity, gelling, solubility and PH.
- Uses: commonly for its thickening and gelling property.
- Safety
- FAQs: is it natural, vegan and the benefits.
What kinds of food labels have you found this ingredient in? Or if you have any questions or remarks about this additive, feel free to let me know in the comments.
Featured image source



But is it harmful to people with stage 3 kidney disease. GFR 40-50?
Is the sodium absorbed, and does it thus increase sodium intake?
Is sodium alginate considered safe in foods for someone on a low iodine diet??